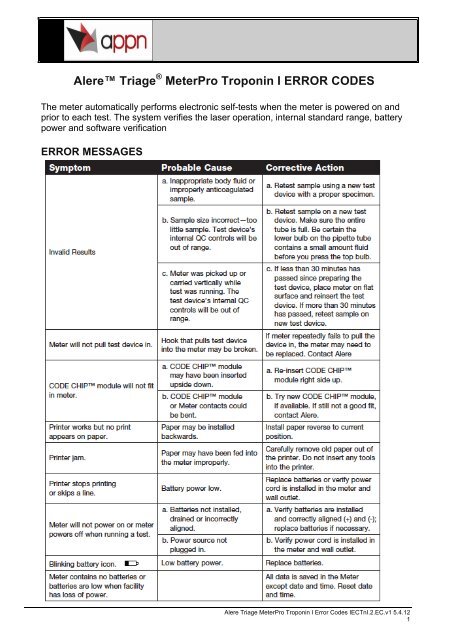
- Triage Meter Pro User Manual Instructions
RALS DEVICE INTERFACE MENU. RALS systems are interfaced to devices you use at the point-of-care every day from market share leaders in glucose, coagulation, blood gas/electrolytes and cardiac markers, and more. Triage ® BNP Test, Alere Triage Cardio2 Panel and Alere Triage Cardio3 Panel devices and the Alere Triage Meter to assist the end user in monitoring product performance. Reagents Alere Triage ® Total 3 Control 1 or Alere Triage Total 3 Control 2 EDTA human plasma containing preservatives, CK-MB, troponin I and B-type natriuretic peptide (BNP.
-
| 510(k) | | | DeNovo | | | Registration & Listing | | | Adverse Events | | | Recalls | | | PMA | | | HDE | | | Classification | | | Standards |
| CFR Title 21 | | | Radiation-Emitting Products | | | X-Ray Assembler | | | Medsun Reports | | | CLIA | | | TPLC |
|
| ALERE SAN DIEGO, INC. TRIAGE METER PRO CARDIAC MARKER TEST | Back to Search Results |
| | Model Number 52111 | | Device Problems Power Cord (497); Smoking (1585); Device Inoperable (1663) | | Patient Problem No Consequences Or Impact To Patient (2199) | | Event Date 02/11/2011 | | Event Type Malfunction | | Event Description | Caller reports power cord to triage barcode scanner was visibly smoking and no longer works. No surges in electricity were observed on site. No explanations for cord damage were offered or source of ignition were found. No personnel were injured or hurt on site. A replacement barcode kit was sent to the customer. Part #52111 corresponds to the replacement barcode kit, which includes the barcode reader and all of its individual accessories. The part number for the triage meter pro is (b)(4). | | Manufacturer Narrative | Investigation pending. | | Search Alerts/Recalls |
|
|

| New Search | Submit an Adverse Event Report |
Triage Meter Pro User Manual Instructions
| Type of Device | CARDIAC MARKER TEST |
|---|
| Manufacturer (Section D) | | ALERE SAN DIEGO, INC. | | san diego CA |
|
|---|
| Manufacturer Contact | | carmen bergelin, manager | | 9975 summers ridge road | | san diego, CA 92121 | | 8588052256 |
|
|---|
| MDR Report Key | 2047391 |
|---|
| MDR Text Key | 19272143 |
|---|
| Report Number | 2027969-2011-00476 |
|---|
| Device Sequence Number | 1 |
|---|
| Product Code | KHO |
|---|
| Combination Product (Y/N) | N |
|---|
| Reporter Country Code | US |
|---|
| PMA/PMN Number | K973547 |
|---|
| Number of Events Reported | 1 |
|---|
| Summary Report (Y/N) | N |
|---|
| Report Source | Manufacturer |
|---|
| Source Type | Other,Health Professional |
|---|
| Reporter Occupation | NOT APPLICABLE |
|---|
| Type of Report | Initial |
|---|
| Report Date | 03/10/2011 |
|---|
| 1 Device Was Involved in the Event |
|---|
| 0 PatientS WERE Involved in the Event: |
|---|
| Date FDA Received | 03/10/2011 |
|---|
| Is This An Adverse Event Report? | No |
|---|
| Is This A Product Problem Report? | Yes |
|---|
| Device Operator | HEALTH PROFESSIONAL |
|---|
| Device MODEL Number | 52111 |
|---|
| Was Device Available For Evaluation? | No |
|---|
| Is The Reporter A Health Professional? | Yes |
|---|
| Date Manufacturer Received | 02/11/2011 |
|---|
| Was Device Evaluated By Manufacturer? | Device Not Returned To Manufacturer |
|---|
| Is The Device Single Use? | Yes |
|---|
| Is this a Reprocessed and Reused Single-Use Device? | No |
|---|
| Type of Device Usage | Unkown |
|---|
|
|

